In the dynamic landscape of pharmaceutical and biotechnology research, true leadership is defined by foresight, a commitment to people, and the courage to redefine industry standards. It’s with this understanding that Enterprise Wired magazine proudly features Catherine “Cathy” Konidas, Chief Administrative Officer at Altasciences, in its “Top Visionary Business Leader to Watch – 2025” issue.
Cathy Konidas embodies a leadership philosophy rooted in empowerment, transparency, and fostering growth. She, alongside the entire executive team at Altasciences, champions a culture where every employee is equipped, informed, and encouraged to contribute—creating an environment of shared purpose and continuous improvement.
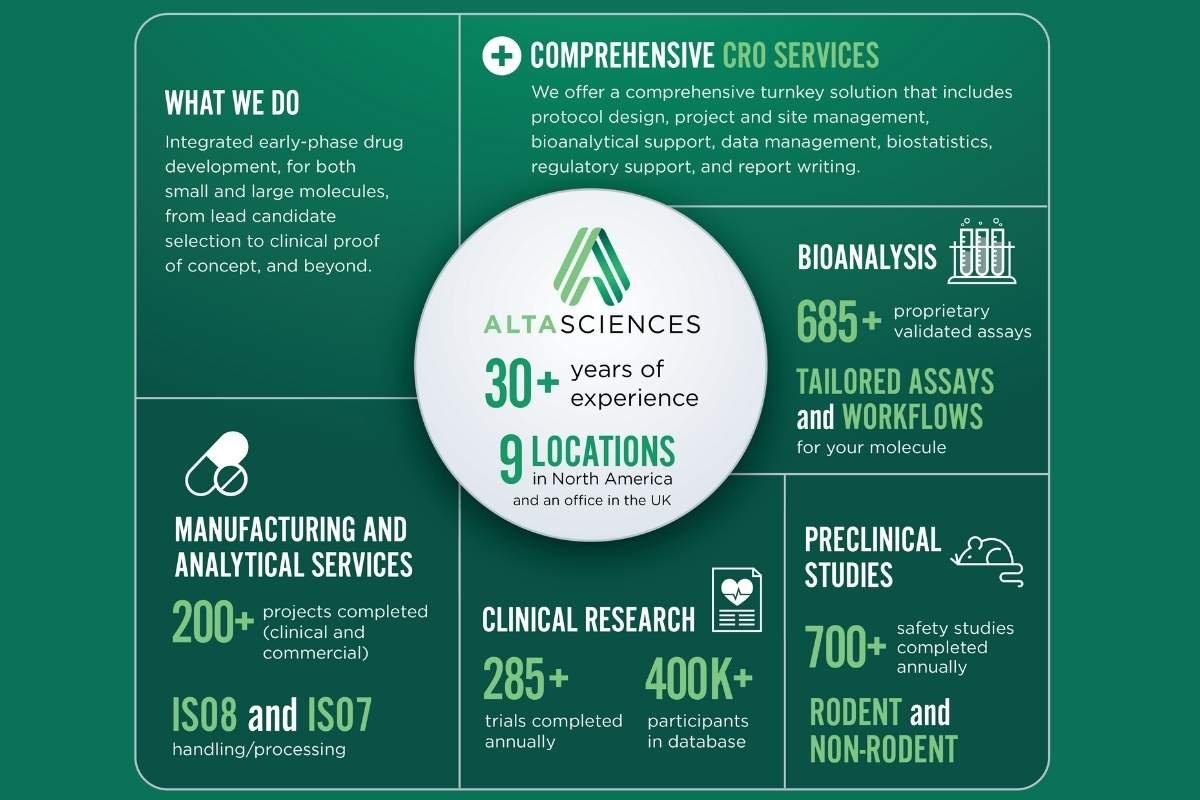
Altasciences has revolutionized the drug development model. By providing integrated, end-to-end solutions, leveraging cutting-edge technology, including AI, and maintaining an unwavering focus on client success, the company can accelerate early-phase R&D timelines by up to 40%. This commitment to efficiency and innovation, combined with a global footprint and a flexible, client-centric methodology, positions Altasciences as a vital partner in helping biopharmaceutical companies bring life-changing therapeutics to patients faster. Join us as we explore what makes Altasciences a different kind of CRO/CDMO, and Cathy Konidas’s vision on leadership and company culture.
Tell us about Altasciences. How has your company been able to disrupt the standard way of outsourcing drug development?
Altasciences is the only CRO/CDMO that can take a sponsor’s molecule from discovery to clinical proof of concept and beyond, seamlessly.
Historically, CROs have functioned as auxiliaries to pharma companies, conducting the work that pharmaceutical and biotechnology companies can’t do in-house. Based on each CRO’s expertise, drug development sponsors have sought partners to complete different aspects or phases of their R&D, and most CROs tend to focus on a specific phase or niche expertise.
In the standard approach, CROs specialize in distinct steps of the drug development stages. This means as a sponsor, you have the additional challenge of working with many different CROs before your drug reaches final regulatory approval.
At Altasciences, we take a different approach, integrating early-phase drug development to deliver a seamless, end-to-end solution. From preclinical research to clinical proof of concept, including clinical pharmacology, CRO services, bioanalysis, formulation, and manufacturing, we centralize it all for streamlined scheduling and communication. The result is true partnership and complete continuity for our clients.
What is your leadership philosophy, and how do you ensure that it is reflected throughout your organization?
As Chief Administrative Officer, my philosophy focuses on empowerment, transparency, open dialogue, and providing growth opportunities for employees, a philosophy that is shared across our entire executive team at Altasciences. So much so that these form the basis for our company’s culture and core values. Empowerment means we ensure our employees have the tools and resources necessary to perform their jobs effectively. In terms of transparency, we make sure we clarify the reasoning behind our business decisions, so that employees understand not only what was decided but also why those decisions were made.

Equally important for me is constructive dialogue: maintaining an open mind and listening to feedback and ideas across the organization, regardless of the person’s rank or role. I always aim to create an environment in which team members feel supported and encouraged to contribute in ways that push Altasciences’ solutions and processes outside of the established norms.
Finally, when someone shows genuine interest and motivation to grow, I believe it’s important to recognize their potential. Talents and passions evolve, and I’ve seen many team members take on roles very different from where they began, simply because they were driven to learn, and we were committed to helping them get there.
How do you identify and develop talent within your organization, and what role do you see mentorship playing in this process?
At Altasciences, we identify talent by looking beyond performance, seeking out individuals who show initiative, adaptability, and a growth mindset. We nurture the potential we see in employees by providing hands-on experience and structured training programs.
Our Leadership Journey Program, for example, was designed to empower Altasciences’ leaders with the tools, insights, and experiences to grow and thrive in their role, and provides a unique development experience for motivated people leaders at every level, from frontline managers to directors.
Our employees regularly learn from each other, from their immediate supervisors, as well as through mentors in the industry and within our organization. I believe that mentorship helps nurture confidence, build perspective, and guide real-time growth. It can include tailored development plans and opportunities for the mentees to lead special projects and expand their skillset.
How has technology helped your customers gain a seamless drug development experience?

Technology has been a key enabler in creating a truly seamless drug development experience for our clients. By integrating proprietary platforms with industry-leading software, we’ve eliminated many of the traditional barriers between study phases. Thanks to our integrated software solutions, sponsors benefit from centralized scheduling and communication, as well as access to data and informed decisions, which lead to optimal timelines and accelerated milestones. The result is a smoother, faster, and more transparent path from preclinical research to clinical proof of concept.
In fact, we have started integrating proven AI tools into our processes and will continue to explore new technologies to enhance workflows. From intuitive dashboards that simplify data review to software that optimizes task allocation, these innovations support us in managing the growing complexity of drug development.
How have you taken the company a step further in terms of capabilities?
In the CRO industry, taking the company “a step further” is critical. It is how we differentiate ourselves in the market, and it begins with listening to our clients and internal teams. Not just to what they say but also to the recurring pain points and inefficiencies that they may not mention, but that we see the impact of. These pain points often signal unmet needs, and when we address them effectively, it creates value for both the client and our business.
By remaining receptive and open-minded, we ensure our services address real needs and deliver meaningful impact in early-stage drug development.
How do you stay innovative and competitive in your industry, and what strategies do you use to adapt to changing market conditions and customer needs?
Staying innovative and competitive requires being nimble and agile in both thought and action. Regardless of our size, we prioritize maintaining flexibility and responsiveness across all aspects of the business. This translates into our strategic approach of building close connections, keeping direct lines of communication open with both our clients and employees. We actively engage with them to understand their evolving needs and challenges, and adapt our strategies accordingly.
This flexibility and responsiveness enable us to refine our offerings, embrace new technologies, and adjust our operations to stay ahead, so that we don’t just meet expectations—we exceed them.
Can you describe a recent successful business expansion, and what factors contributed to its success?
In just the last five years, we’ve taken deliberate, strategic steps to expand Altasciences’ reach and capabilities, always with our clients’ evolving needs at the center. Recognizing the growing demand for preclinical capacity, we added three new preclinical facilities across the U.S., bringing our total sites to four, and strengthening our clinical footprint by opening a third clinical pharmacology unit.
To further streamline the drug development journey, we acquired a CDMO facility in Philadelphia, extending our integrated model from preclinical through clinical proof of concept, including manufacturing. Our success comes down to foresight, agility, and a clear vision: to stay ahead of the curve so our clients can move their molecules forward with speed and confidence.
Conclusion
Cathy Konidas exemplifies the philosophy that drives Altasciences forward: stay open to opportunity, but grounded in thoughtful execution. Her approach combines smart risk-taking with rigorous planning and open communication, ensuring that innovation is always matched by operational discipline. Altasciences continues to evolve, expanding capabilities, refining processes, and setting new expectations for what a CRO/CDMO can deliver. It’s a strategy defined not just by vision, but by action, and one that keeps the company, and its clients, ahead of the curve.










